Get the latest information from the FDA to help you make an informed decision about using or not breast implants.
What should I get breast implants? What should be saline or silicone? What style? How much monitoring is needed after surgery?
These are common questions that people have when they consider breast implants.
That is why the Food and Drug Administration of usa. UU. (FDA, for its acronym in English) offers the following information (in English) to help people make decisions about breast implants.
The FDA has approved implants to increase the size of the breasts, for reconstruction after surgery or trauma cause of breast cancer, and to correct developmental defects. The FDA has also approved breast implants to correct or improve the result of a prior surgery.
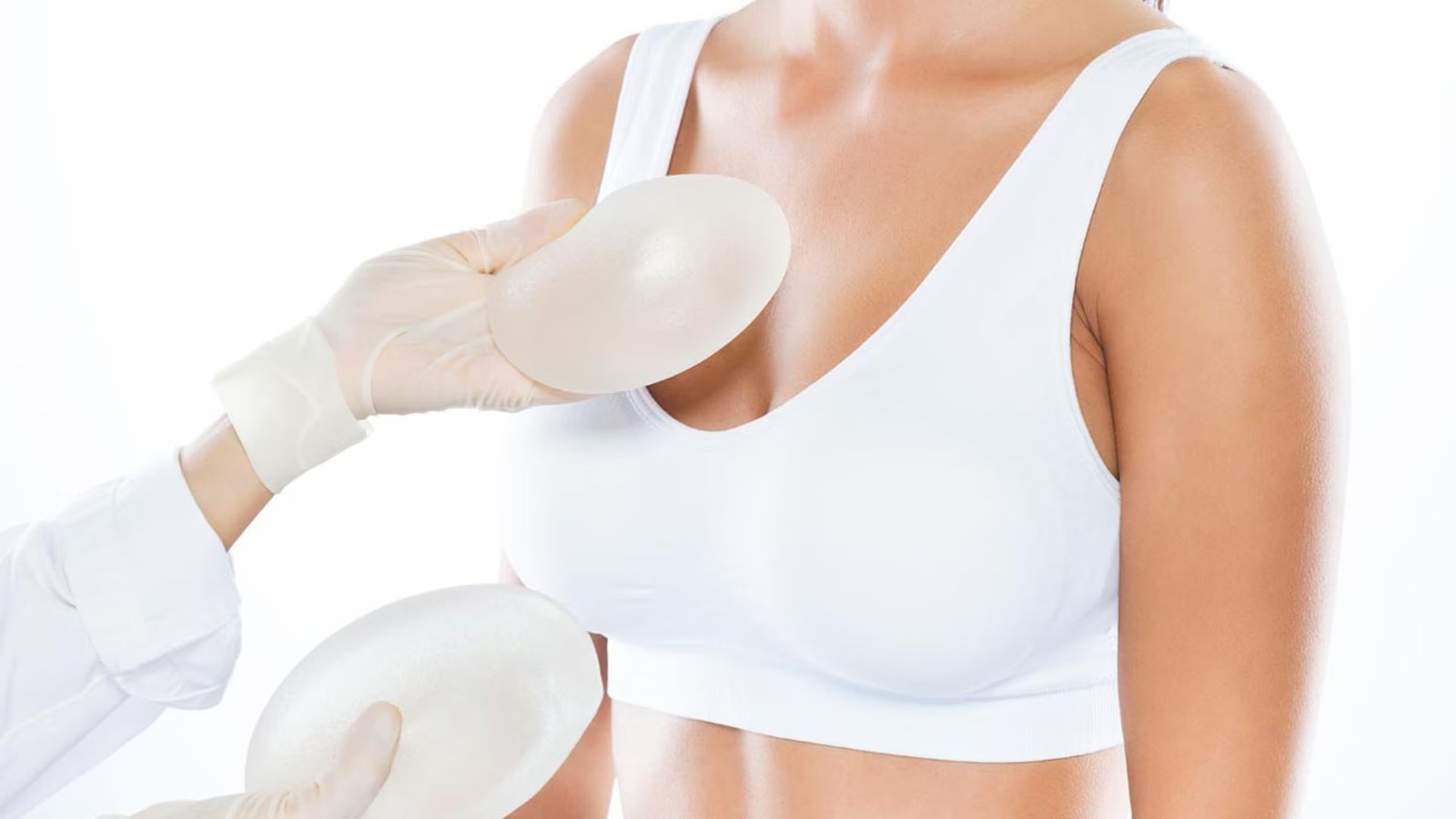
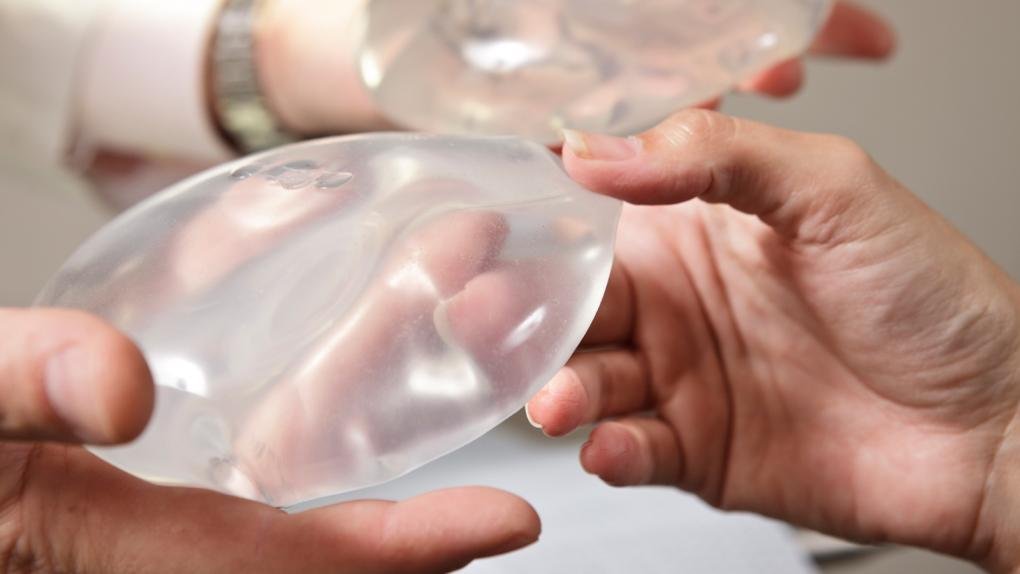
There are two types of breast implants approved by the FDA: the filled with saline (salt water solution) and the silicone gel-filled. Both have an outer silicone and vary in size, thickness of the cover, the texture of the surface of the cover and shape (contour).
Know the risks of breast implants
The implants approved by the FDA are subject to extensive testing before approval in order to demonstrate a reasonable assurance of safety and effectiveness. Even so, there are risks associated with all breast implants (in English), including:
- Additional surgeries
- Anaplastic large cell lymphoma associated with breast implants (BIA-ALCL, for its acronym in English) (in English), which is a cancer of the immune system
- Systemic symptoms, commonly referred to as Disease of the breast implants (BII, for its acronym in English)
- Capsular contracture (scar tissue that squeezes the implant)
- Pain in the breasts
- Rupture (tearing or holes in the deck) of the saline-filled implants and silicone gel
- Disinflation (with visible changes in the size of the breasts) of the saline-filled implants
- Breaking silent (no symptoms) of implants filled with silicone gel
- Infection
The silicone used in breast implants is different to the silicone injection. The silicone injection is not approved by the FDA for use in breast augmentation, breast reconstruction or body contour.
Breast implants are not lifetime devices
The more time you have breast implants, the greater the chances that you will develop complications, some of which will require more surgery.
The lifespan of breast implants varies from person to person and cannot be predicted. That means that all people with breast implants may need additional surgeries, but no one can predict when. Patients may also request additional surgeries to modify the size or shape of their breasts.
Understand the labels of the implants
To help patients to be aware of the risks of breast implants prior to surgery, the FDA requires that all breast implants approved by the FDA include labels with easy to understand information about the benefits and risks associated with the product. This requirement aims to enhance the conversations that patients have with their doctors about the benefits and risks of the implants. The mandatory information on the label includes a check list of the decisions of the patient, which highlights the key information on risks; warning information in the form of a box to make it visible; and a card of the device to the patient with specific information of the implant from the patient, such as the serial or lot number, and the style and size.
The FDA restricts the sale and distribution of breast implants just to the health care providers and facilities that provide information to patients using the check list of decisions of the patient. The patient should have the opportunity to put their initial and sign the check list before agreeing to the surgery and must be signed by the physician who implanted the device.
In addition, the FDA advises patients to read the Summary of safety and efficacy data (in English) and the label of the product of each implant to get to know the materials used to build the device, the characteristics of the same and the fillers used. The Summary of safety and efficacy data has been developed for all breast implants saline and silicone approved. The Summary of safety and efficacy data and the product label provides information on the indications for use, risks, warnings, precautions, and studies associated with the approval of the device by the FDA.
The Summary data of the safety and efficacy also provides information about the clinical study used to the approval of the device. More serious complications are those that lead to new surgeries, such as rupture or capsular contracture, or the diagnosis of BIA-ALCL.
Talk with your surgeon
The surgeons will assess the shape, size, surface texture, the placement of the implant and the place of the incision for each person. Ask your surgeon about his experience in the realization of surgery of breast implants, the surgical procedure and the ways in which the implant may affect your life.
Report to your surgeon about previous surgeries and the response of your body. For example, mention if the surgeries resulted in an amount of scar tissue was higher than expected. Also, talk about your expectations. These conversations will help you and the surgeon to make decisions to achieve the desired look, including the decisions on the location and the size of the incision, as well as the size, material, texture, and placement of the implant.
Many patients undergo additional operations to change the size of the implant. To achieve the best results after the first procedure, it is necessary a careful planning and reasonable expectations.
Get to know the long-term risks of breast implants
The FDA has identified an association between breast implants and the development of anaplastic large cell lymphoma associated with breast implants (BIA-ALCL), a type of non-hodgkin lymphoma. Patients with breast implants may have a higher risk of developing this cancer, which develops mainly in the fluid or scar tissue surrounding the implant.
Breast implants have smooth surfaces or textured (covered). The BIA-ALCL develops more frequently in patients with textured implants than in those with implants with a smooth surface. Like other lymphomas, the BIA-ALCL is a cancer of the immune system and not of the breast tissue.
Some patients with implants have also reported health problems, such as connective tissue diseases (such as lupus and rheumatoid arthritis), breastfeeding problems, or reproductive problems. Currently, there is sufficient evidence to support an association between breast implants and these diagnoses.
In addition, some patients who received breast implants have reported a variety of systemic symptoms such as joint pain, muscle aches, confusion, chronic fatigue, autoimmune diseases, and others. The individual risk of patients to develop these symptoms has not been well established.
Monitor your breast implants
In general, follow your surgeon's instructions on how to monitor your breast implants.
If you notice any signs or unusual symptoms, report them immediately to your surgeon or health care provider. It is recommended that health care providers and patients to report adverse events or side effects related to the use of these products Program of Information Security, and Reporting of Adverse Events to the FDA's MedWatch (in English).
In addition, follow the instructions of your health care provider for the imaging for the detection of breast cancer, as this may be different for patients who underwent a breast augmentation, and for patients who underwent breast reconstruction. If you are going to schedule an appointment for a mammogram, report to the mammography facility that you have breast implants and ask them what you can expect with respect to the mammogram with breast implants.
Your surgeon or health care provider may also recommend other tests, such as ultrasound or magnetic resonance imaging (MRI, for its acronym in English). The FDA recommends that patients with silicone implants to conduct periodic examinations to detect ruptures are silent.
If you have specific questions about breast implants, consult with your surgeon or health care provider.
Get the latest information from the FDA to help you make an informed decision about whether or not to use breast implants.
Should I get breast implants? Should they be saline or silicone? What style? How much monitoring is needed after surgery?
These are common questions people have when considering breast implants.
That’s why the U.S. Food and Drug Administration (FDA) offers the following information to help people make decisions about breast implants.
The FDA has approved implants to increase breast size, for reconstruction after surgery or trauma due to breast cancer, and to correct developmental defects. The FDA has also approved breast implants to correct or improve the outcome of a previous surgery.
There are two types of FDA-approved breast implants: those filled with saline (saltwater solution) and those filled with silicone gel. Both have a silicone outer shell and vary in size, shell thickness, shell surface texture, and shape (contour).
Learn About The Risks Of Breast Implants
FDA-approved implants undergo extensive testing before approval to demonstrate reasonable assurance of safety and effectiveness. Still, there are risks associated with all breast implants , including:
- Additional surgeries
- Breast implant-associated anaplastic large cell lymphoma (BIA-ALCL) , which is a cancer of the immune system
- Systemic symptoms, commonly referred to as Breast Implant Illness (BII)
- Capsular contracture (scar tissue that squeezes the implant)
- Pain in the breasts
- Rupture (tears or holes in the shell) of saline-filled and silicone gel-filled implants
- Deflation (with visible change in breast size) of saline-filled implants
- Silent rupture (without symptoms) of silicone gel-filled implants
- Infection
The silicone used for breast implants is different than injectable silicone. Injectable silicone is not FDA approved for breast augmentation, breast reconstruction, or body contouring.
Breast Implants Are Not Lifelong Devices
The longer you have breast implants, the greater the chances that you will develop complications, some of which will require more surgery.
The lifespan of breast implants varies from person to person and cannot be predicted. This means that all people with breast implants may need additional surgeries, but no one can predict when. Patients may also request additional surgeries to modify the size or shape of their breasts.
Understanding Implant Labels
To help patients be aware of the risks of breast implants before surgery, the FDA requires that all FDA-approved breast implants include labels with easy-to-understand information about the benefits and risks associated with the product. This requirement is intended to improve the conversations patients have with their physician about the benefits and risks of implants. Required label information includes a patient decision checklist that highlights key risk information; boxed warning information for visibility; and a patient device card with information specific to the patient’s implant, such as serial or lot number, and style and size.
The FDA restricts the sale and distribution of breast implants only to health care providers and facilities that provide information to patients using the Patient Decision Checklist. The patient must have the opportunity to initial and sign the checklist before agreeing to surgery, and it must be signed by the physician implanting the device.
Additionally, the FDA advises patients to read the Summary of Safety and Effectiveness Data (SED) and product labeling for each implant to learn about the materials used to construct the device, the characteristics of the device, and the fillers used. The SED has been developed for all approved saline and silicone breast implants. The SED and product labeling provide information about the indications for use, risks, warnings, precautions, and studies associated with the FDA’s approval of the device.
The Safety and Efficacy Data Summary also provides information about the clinical study used for the approval of the device. The most serious complications are those that lead to further surgery, such as rupture or capsular contracture, or the diagnosis of BIA-ALCL.
Talk To Your Surgeon
Surgeons will evaluate the shape, size, surface texture, implant placement, and incision site for each individual. Ask your surgeon about his or her experience performing breast implant surgeries, the surgical procedure, and the ways the implant might affect your life.
Tell your surgeon about previous surgeries and your body’s response. For example, mention whether the surgeries resulted in more scar tissue than expected. Discuss your expectations, too. These conversations will help you and your surgeon make decisions to achieve your desired appearance, including decisions about incision location and size, as well as implant size, material, texture, and placement.
Many patients undergo additional operations to change the size of the implant. To achieve the best results after the first procedure, careful planning and reasonable expectations are necessary.
Learn About The Long-Term Risks Of Breast Implants
The FDA has identified an association between breast implants and the development of breast implant-associated anaplastic large cell lymphoma (BIA-ALCL), a type of non-Hodgkin lymphoma. Patients with breast implants may be at increased risk of developing this cancer, which develops primarily in the fluid or scar tissue surrounding the implant.
Breast implants have either smooth or textured (coated) surfaces. BIA-ALCL develops more often in patients with textured implants than in those with smooth-surfaced implants. Like other lymphomas, BIA-ALCL is a cancer of the immune system and not of breast tissue.
Some patients with implants have also reported health problems such as connective tissue diseases (such as lupus and rheumatoid arthritis), breastfeeding problems, or reproductive problems. Currently, there is insufficient evidence to support an association between breast implants and these diagnoses.
In addition, some patients who received breast implants have reported a variety of systemic symptoms such as joint pain, muscle aches, confusion, chronic fatigue, autoimmune diseases, and others. Individual patients’ risk of developing these symptoms has not been well established.
Monitor Your Breast Implants
In general, follow your surgeon’s instructions on how to monitor your breast implants.
If you notice any unusual signs or symptoms, report them immediately to your surgeon or health care provider. Health care providers and patients are encouraged to report adverse events or side effects related to the use of these products to the FDA’s MedWatch Safety Information and Adverse Event Reporting Program .
Also, follow your healthcare provider’s instructions for breast cancer screening imaging, as this may be different for patients who have had breast augmentation and patients who have had breast reconstruction. If you are scheduling an appointment for a mammogram, tell the mammography facility that you have breast implants and ask them what you can expect regarding mammography with breast implants.
Your surgeon or health care provider may also recommend other tests, such as ultrasound or magnetic resonance imaging (MRI). The FDA recommends that patients with silicone implants have regular checkups to detect silent ruptures.
If you have specific questions about breast implants, consult your surgeon or health care provider.
U. S. Food & Drugs Administration
09/08/2022
Original Source: https://www.fda.gov/consumers/articulos-para-el-consumidor-en-espanol/que-debe-saber-sobre-los-implantes-de-seno